Friday, July 31, 2020
Study Shows Cannabis Provides Relief To 9 Out Of 10 Migraine Patients
from News – High Times https://ift.tt/33eycjO
via IFTTT
House Votes To Block Federal Interference Of Legal Cannabis
from News – High Times https://ift.tt/2BK5UCe
via IFTTT
Where’s California’s Hemp Production Plan?

The Agriculture Improvement Act of 2018 (2018 Farm Bill) legalized hemp by removing the crop and its derivatives from the definition of marijuana under the Controlled Substances Act (CSA) and by providing a detailed framework for the cultivation of hemp. The 2018 Farm Bill gives the US Department of Agriculture (USDA) regulatory authority over hemp cultivation at the federal level. In turn, states have the option to maintain primary regulatory authority over the crop cultivated within their borders by submitting a plan to the USDA.
While states are free to submit plans at any time, the clock is essentially ticking since the prior federal law governing hemp cultivation (the Agriculture Act of 2014 or “2014 Farm Bill”) will expire on October 31, 2020 per interim rules released by the USDA last year. What this means is that states who fail to submit hemp production plans and have them approved by October 31 could cause problems for their internal hemp industries that may be inconsistent with federal law.
That brings me to California. Last year, the state passed a law (SB-153) that mandated that the state submit a hemp production plan to the USDA by no later than May 1, 2020. Specifically, current California law says:
On or before May 1, 2020, the secretary, in consultation with the Governor and the Attorney General, shall develop and submit to the United States Secretary of Agriculture a state plan, consistent with this division, pursuant to Section 297B of the federal Agricultural Marketing Act of 1946 (added by Section 10113 of the federal Agriculture Improvement Act of 2018 (Public Law 115-334)), including a certification that the state has the resources and personnel to carry out the practices and procedures described in clauses (i) to (iv), inclusive, of subparagraph (A) of paragraph (2) of subsection (a) of that section.
Translated to English, the California Department of Food and Agriculture (CDFA) and California Attorney General (AG) were supposed to work together to get a plan submitted by May 1. That didn’t happen and even the USDA’s website notes that it’s still waiting for the plan.
So what happened? Well, according to the CDFA’s FAQs, “California is in the process of developing a state plan, and thus, California hemp growers are not currently subject to the federal interim rule. However, growers in states that do not have a pending or approved regulatory plan may apply for a USDA hemp production license.” Additionally, in early July, CDFA sent an email blast that read in part:
The Department’s draft state regulatory plan for hemp production was completed prior to May 1, 2020, however state law requires the Department to submit it to both the Attorney General’s Office and Governor’s Office for review and approval. We are currently awaiting their comments after which we will submit the plan to USDA. As you can understand, both have had a lot on their plates dealing with the pandemic, as have we all.
Basically, CDFA is apparently still waiting on the AG and Governor to review the plan. What that means for the California hemp industry is not clear. Once the 2014 Farm Bill expires, per the CDFA’s own guidance and guidance issued by the USDA, growers may need to apply for federal hemp production licenses. That seems completely overkill, because once California’s plan is adopted, those federal licenses would become unnecessary. Ultimately, only time will tell what will happen with the state’s plan once it is submitted.
Federal law is clear that the USDA will have 60 days to review California’s hemp production plan once it is submitted. If California wants to beat that October 31 deadline, it needs to get its plan submitted, and to do so fast. Stay tuned to the Canna Law Blog for more updates.
The post Where’s California’s Hemp Production Plan? appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/2Xi5lY6
via IFTTT
Thursday, July 30, 2020
Did women use cannabis as medicine in ancient Egypt?
While we don’t know everything about this ancient empire, we do know women worked with medicine and magic, and that cannabis may have played a part.
The post Did women use cannabis as medicine in ancient Egypt? appeared first on Leafly.
from Leafly https://ift.tt/2PaLx4m
via IFTTT
Maine Officials Predict Start of Recreational Cannabis Sales by End of Year
from News – High Times https://ift.tt/312dZLp
via IFTTT
Best cannabis subscription boxes
A BirchBox of buds. A FabFitFun of flower. It’s a thing!
The post Best cannabis subscription boxes appeared first on Leafly.
from Leafly https://ift.tt/2pOkM7y
via IFTTT
Rapper Loon Released from Prison After Nine Years Serving Non-Violent Drug Charge
from News – High Times https://ift.tt/3hL8uau
via IFTTT
Oregon Cannabis Litigation: Cura Hit with $10 Million Class Action

It has been a tough stretch for Portland-based Cura Partners, Inc. (“Cura”) and its parent company, Massachusetts-based Curaleaf Holdings, Inc. (“Curaleaf.”)
Cura produces the popular Select brand of THC vaping products and was declared Oregon’s first “cannabis unicorn.” In May of 2019, Cura announced its sale to Curaleaf for more than $1 billion in an all-stock transaction. Around the same time, Curaleaf announced it would pay some $875 million, mostly in stock, to acquire a Chicago based cannabis company, Grassroots. These deals made Curaleaf one the world’s largest marijuana companies. Sounds good right?
It didn’t last long. One year ago today, Curaleaf was hit with an FDA warning letter for “illegally selling” CBD products and making health claims about those products. That was followed by a class action securities lawsuit alleging that Curaleaf made knowingly false statements to the investing public. Shortly after, Curaleaf was fined $250,000 by the State of Massachusetts for failing to disclose change of ownership to state regulators. You can find our comprehensive analysis on that brutal stretch here.
Things don’t seem to have improved much for the Curaleaf family of companies in 2020. In January, Cura paid a record $110,000 fine for mislabeling products in Oregon, and found itself defending a class-action lawsuit alleging it mislabeled cannabis products. In February, the vaunted $1 billion Curaleaf deal finally closed, but for a cash payment of $285 million for 55 million shares, and other incentive bonuses. By March, Curaleaf’s shares had dropped to $2.75/per, down from $6.31/per to start off the year.
Although the share price has since recovered to match last July’s peak, last week another large lawsuit was filed against Cura, Curaleaf’s newly minted acquisition. This one is $10 million class action, styled as Blackford v. Cura CS, LLC, No. 20CV25203. The lawsuit was filed on the same day that Curaleaf announced the closure of its deal with Grassroots for approximately $830 million. (Email me if you’d like a copy of the complaint.)
The gravamen of the complaint is the allegation that the Select Elite brand of THC vape products “do not contain anywhere near the quantities of THC advertised.” According to the plaintiff, independent lab testing reveals that the Select Elite products contained only 55% THC despite promising (on labels and other advertising) that they would contain 76.9% THC. Industry watchers know that mislabeling is a common problem in the cannabis industry, but 76.9% to 55% is quite a spread.
Plaintiff seeks relief individually, and as a class action on behalf of similarly situated purchasers of Cura’s products, for: (i) breach of express warranty; (ii) breach of the implied warranty of merchantability; (iii) unjust enrichment; (iv) fraud; and (v) violation of Oregon’s Unlawful Trade Practices Act (“UTPA”), ORS 646.605, et seq. These are the usual claims in any class action; notably, the trade practices claim permits an award of attorneys’ fees as well as statutory damages. (See here).
Plaintiff seeks to certify a class comprised of all persons who purchased Select Elite THC Products in Oregon. But this lawsuit may be just the beginning, as Cura sells its Select Elite products in more than 900 dispensaries, including in California. I would expect enterprising plaintiff’s attorneys to look into similar actions in other states where Select Elite products are sold.
Over the years, we have seen similar class action lawsuits concerning CBD products to the lawsuit filed last week against Cura. It is no surprise that these lawsuits target the largest players in the hemp and marijuana industries. (See here). But class action lawsuits are not confined to multi-state operators, particularly in states where unfair trade practices law allows a plaintiff to seek her attorneys’ fees. Anyone involved in the manufacture or sale of THC products is a potential defendant and at risk of not just civil lawsuits, but action by state regulators.
The post Oregon Cannabis Litigation: Cura Hit with $10 Million Class Action appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/314TKN5
via IFTTT
Wednesday, July 29, 2020
Mississippi State Leaders Urge Voters To Consider Medical Marijuana Proposals Carefully
from News – High Times https://ift.tt/2P4bEtG
via IFTTT
Nevada Dispensaries Accused of Selling Tainted Cannabis
from News – High Times https://ift.tt/3jV69eU
via IFTTT
Police Raid Five Unlicensed Dispensaries In San Diego County
from News – High Times https://ift.tt/3gabnkY
via IFTTT
Election 2020: Oregon psilocybin and drug decriminalization initiatives guide
Oregon voters will consider one measure to legalize the medical use of psilocybin, and another to decriminalize small amounts of all drugs.
The post Election 2020: Oregon psilocybin and drug decriminalization initiatives guide appeared first on Leafly.
from Leafly https://ift.tt/3ffsxMC
via IFTTT
Election 2020: Arizona marijuana legalization initiative guide
Some of the donors who defeated Arizona's 2016 legalization bid are now in prison. 2020 could be the year Arizonans finally get it done.
The post Election 2020: Arizona marijuana legalization initiative guide appeared first on Leafly.
from Leafly https://ift.tt/2D2Pk13
via IFTTT
Election 2020: Montana cannabis legalization guide
Montana advocates overcame a COVID-19 shutdown to put adult-use cannabis legalization on the Nov. 2020 ballot. Early polls are favorable.
The post Election 2020: Montana cannabis legalization guide appeared first on Leafly.
from Leafly https://ift.tt/3i0tNVV
via IFTTT
Election 2020: Mississippi medical marijuana legalization guide
Initiative 65 would legalize the use of medical marijuana. A rival measure, 65A, would derail the whole dang thing.
The post Election 2020: Mississippi medical marijuana legalization guide appeared first on Leafly.
from Leafly https://ift.tt/39CRf8w
via IFTTT
Election 2020: Idaho medical marijuana legalization guide
Everything you need to know about Idaho's medical marijuana legalization initiative, on the Nov. 2020 ballot—maybe.
The post Election 2020: Idaho medical marijuana legalization guide appeared first on Leafly.
from Leafly https://ift.tt/30SkTT6
via IFTTT
Election 2020: New Jersey cannabis legalization guide
If voters approve a constitutional amendment on Nov. 3, New Jersey will become the first mid-Atlantic state to legalize cannabis for all adults.
The post Election 2020: New Jersey cannabis legalization guide appeared first on Leafly.
from Leafly https://ift.tt/2BF9lKs
via IFTTT
Election 2020: South Dakota cannabis legalization guide
South Dakota voters could make history on Nov. 3 by becoming the first state to pass both medical and adult-use legalization on a single day.
The post Election 2020: South Dakota cannabis legalization guide appeared first on Leafly.
from Leafly https://ift.tt/333xBBn
via IFTTT
Election 2020: Nebraska medical marijuana legalization guide
Medical legalization enjoyed 77% support in a statewide poll a few years ago. Will Nebraska voters make it a reality on Nov. 3?
The post Election 2020: Nebraska medical marijuana legalization guide appeared first on Leafly.
from Leafly https://ift.tt/39BTRDw
via IFTTT
COVID-19 and medical marijuana patients: What you need to know
During the COVID-19 crisis, states are opening up online ordering and delivery options so medical patients can get marijuana safely. Check out what your state's doing.
The post COVID-19 and medical marijuana patients: What you need to know appeared first on Leafly.
from Leafly https://ift.tt/2Jm1668
via IFTTT
Federal Policy on Hemp CBD Is Taking Shape: What Needs to Be Addressed?
This is the first post in a two-part series.
Last week, the Food and Drug Administration (FDA) submitted a CBD enforcement policy to the White House. We do not yet have the text of that document but we anticipate that it will have a significant impact on hemp-derived CBD (Hemp CBD) products.
In anticipation of the release of the FDA’s CBD enforcement policy, we wanted to cover the FDA’s current position on Hemp CBD, which is summarized in the following graphic:
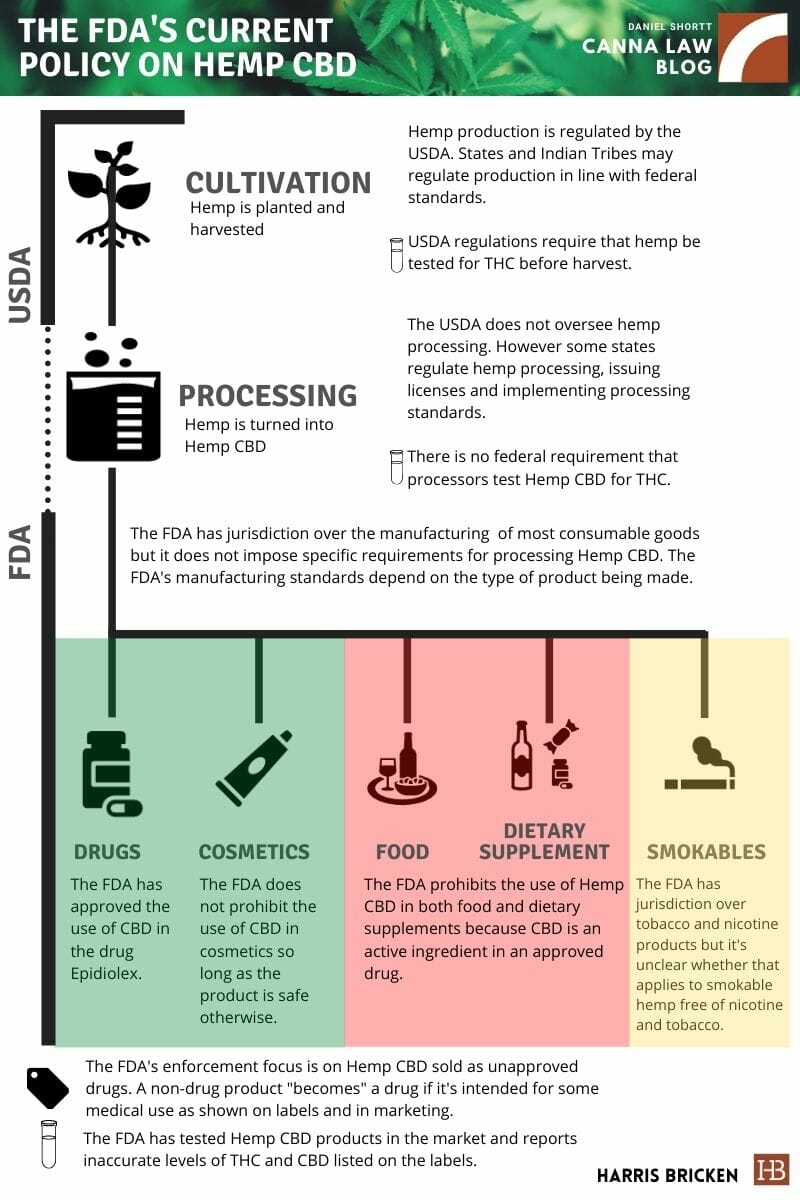
As you can see, the FDA has some things to address. The most pressing issue is the treatment of foods and dietary supplements, which are common in the marketplace but are allegedly prohibited by the FDA. The FDA has done very little to actually prevent the sale of Hemp CBD in food and dietary supplements. It also has not provided any interim guidance on processing hemp into cosmetics, foods, dietary supplements, or smokable products.
What the FDA has done instead is send warning letters about Hemp CBD products of all types (not just food and dietary supplements) that are marketed as drugs based on the use of health or medical claims. The FDA also recently released guidance on how drug manufacturers (i.e., those who knowingly are making drugs not other products that are then marketed as drugs) can research cannabis derivates.
One feature of the FDA drug guidance that could show up in the FDA enforcement guidance submitted to the White House is the instruction on how to measure THC in hemp products. As indicated in the above graphic (with a test tube icon) there are not well-established testing standards for hemp products. The 2018 Farm Bill and USDA cover the testing of hemp during the cultivation stage but not the testing of hemp derivatives during the processing stage or hemp products sold in commerce. In part two of this series, I’ll discuss the issue of testing Hemp CBD products.
The post Federal Policy on Hemp CBD Is Taking Shape: What Needs to Be Addressed? appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/3jQgclE
via IFTTT
Tuesday, July 28, 2020
New Poll From Law Firm Shows Cannabis Legalization Is Popular Among New Jersey Voters
from News – High Times https://ift.tt/3g7gBha
via IFTTT
Martha Stewart Accepts Challenge to Smoke a Joint with Chelsea Handler
from News – High Times https://ift.tt/2P0qtxk
via IFTTT
Democrats Decline To Include Cannabis Legalization In Party Platform
from News – High Times https://ift.tt/30UuFV0
via IFTTT
Clint Eastwood Sues CBD Companies for Unauthorized Use of Name and Likeness

On July 22, 2020, Clint Eastwood filed two separate lawsuits in federal court in Los Angeles against three CBD manufacturers and marketing companies that posted fabricated online articles falsely claiming that Eastwood endorsed CBD products, as well as ten online CBD retailers that allegedly manipulated search results to make it appear that he had endorsed their products. The court filings assert that “Mr. Eastwood has no connection of any kind whatsoever to any CBD products and never gave such an interview.”
According to the New York Times article on the lawsuits, the bizarre fake news stories claimed that Eastwood endorsed their products and was leaving filmmaking to focus on the CBD business. These same companies allegedly send spam emails with subject lines like, “Clint Eastwood Exposes Shocking Secret Today,” and containing a fake interview with an outlet intended to look like the “Today” show.
The second lawsuit against the online retailers claims that those retailers are “using programming code to insert Eastwood’s name into online search results for CBD products, misleading consumers into thinking the filmmaker is manufacturing or endorsing them.” This is unfortunately not the first time that unscrupulous CBD companies have attempted to falsely claim that celebrities have endorsed their products.
And of course, improperly using the names and likenesses of celebrities to promote product sales is a tactic that companies have attempted to use for as long as celebrities have existed. This is why in California, we have a body of law around the “Right of Publicity,” which protects against the unauthorized use of a person’s name or likeness for commercial or exploitative purposes. This body of law is based both in common law and in statute. Under Cal. Civ. Code § 3344:
“[a]ny person who knowingly uses another’s name, voice, signature, photograph, or likeness, in any manner, on or in products, merchandise, or goods, or for purposes of advertising or selling, or soliciting purchases of, products, merchandise, goods or services, without such person’s prior consent, or, in the case of a minor, the prior consent of his parent or legal guardian, shall be liable for any damages sustained by the person or persons injured as a result thereof.”
In interpreting the statute, courts have utilized a three-part test to determine whether there has been a violation of the statute:
- Was there a “knowing” use of the plaintiff’s protected identity?
- Was the use for advertising purposes?
- Was there a direct connection between the use of the plaintiff’s identity and the commercial purpose?
Based on the facts alleged in Eastwood’s complaint, if those facts are true, he appears to have a good argument that these companies violated the statute and his rights of publicity. Under the common law, a plaintiff must allege four things to prove there has been a violation of the common law right of publicity:
- Defendant’s use of plaintiff’s “identity”;
- Appropriation of plaintiff’s name or likeness to defendant’s advantage, commercially or otherwise;
- Lack of plaintiff’s consent; and
- Resulting injury.
Celebrity endorsement has been a popular means for promoting both cannabis and hemp-CBD products for the last several years. Companies seeking to make use of a celebrity’s name or likeness in promoting their products should be aware of the complexities of negotiating and implementing these types of deals, of which I have handled many.
Of course, it goes without saying that using a celebrity’s name or likeness without their consent is certain to land you in hot water for misuse of their right of publicity. If your brand intends to make any insinuation that your product is approved by, endorsed by, or associated with any individual (living or dead), consult with your cannabis IP attorney first.
The post Clint Eastwood Sues CBD Companies for Unauthorized Use of Name and Likeness appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/39wWHK8
via IFTTT
Monday, July 27, 2020
Is it safe to consume cannabis during and after pregnancy?
Check out Leafly's comprehensive guide on cannabis and pregnancy and see what the science has to say.
The post Is it safe to consume cannabis during and after pregnancy? appeared first on Leafly.
from Leafly https://ift.tt/3f6HKPS
via IFTTT
Colorado Springs Reconsidering Adult-Use Cannabis
from News – High Times https://ift.tt/2CSuvW9
via IFTTT
$6 Million Worth of Cannabis Seized At New York-Canada Border
from News – High Times https://ift.tt/2DcUfw2
via IFTTT
Oregon Cannabis: Recent OLCC Settlement Trends

We regularly represent marijuana businesses that find themselves on the wrong side of an OLCC charging document. The charging document will list the particular rule or rules that the OLCC alleges the licensee has violated and always, in my opinion, lists only the most severe sanctions.
The OLCC sanctions schedule is laid out in tiers. For Category I violations, the default sanction is license cancellation. The default sanctions for lesser violations are lower. For example, the standard sanction for a Category II(b) violation is a 30-day license suspension or a civil penalty of $4,950. The standard sanction for a first-level Category III violation is a 10-day suspension or $1,650 civil penalty. The proposed sanctions cascade when the charging document includes several violations, or when the licensee has been in trouble with the OLCC before.
The first questions a new client usually asks are: Does the OLCC really intend to cancel my license? Is a settlement for something less than cancellation possible? How long can I operate my business before my license is cancelled? The answer to these questions always depends on the facts and circumstances that led to the OLCC issue a charging document.
One way to evaluate settlement prospects is to look at past settlements between the OLCC and a licensee. This also useful when commencing negotiations with the OLCC Case Presenter (which is the title for an OLCC attorney assigned to the handle the administrative proceeding). Settlements are the norm and are negotiated between the OLCC Case Presenter and the licensee or its attorney. The “higher ups” at the OLCC often provide the Case Presenter guidance and review all potential settlements of Category I violations for less than license cancellation. Once terms are reached, the proposed settlement goes before the Commissioners at one of their monthly meetings for approval.
Past settlements are available to the public on the OLCC’s website. Here is brief synopsis of some of those settlements in the past few months:
| General Description of Violation | Category | Settlement |
| A site inspection revealed that the licensee had failed to accurately record harvest information. On two occasions, licensee failed to segregate harvest lots within 45 days of harvest. Licensee also had discrepancies between what was listed in CTS and its physical inventory for one package, and harvested marijuana could not be located on the premises during the inspection. | Category III (2) | Licensee will pay a $2,640.00 civil penalty June 15, 2020
OR serve a 16-day suspension |
| Licensee, who held a producer license, was discovered to have engaged in the transfer of marijuana product between two different producer licensees, which is prohibited under the rules. During the course of the investigation into the transfers, it was discovered that Licensee also had an ODA-registered hemp grow on the same tax lot as the licensed premises, but failed to submit a hemp control plan to the Commission for approval. Licensee subsequently obtained Commission approval for a hemp control plan. | Category I (2)
|
Licensee will either pay a $4,950.00 civil penalty before 5:00 PM AND serve a 60-day suspension OR pay a $9,900.00 civil penalty AND serve a 30-day license suspension |
| Licensee was improperly growing hemp on the licensed premises, and had hemp products on-site. Licensee had also altered the premises by adding a greenhouse, offices, and a storage container without obtaining approval from the Commission beforehand. Licensee’s prior compliance officer had falsely represented to Licensee that the appropriate forms had been submitted and approved by the Commission. When Licensee discovered the problems, they were rectified soon after. | Category I
Category III |
Licensees will pay a $6,105.00 civil penalty OR serve a 37-day license suspension |
| OLCC Dispatch received a complaint of an explosion at the premises. Investigation revealed that this producer Licensee was processing hemp without either a processor license or a hemp endorsement. The lack of a processor license meant that the processing equipment had not been safety-certified as required as part of the OLCC pre-licensing process. Fortunately there was no report of serious injury. | Category I (2) | License cancellation, though the Licensee was permitted to try and sell the business. |
| Investigations on five of Licensee’s producer premises were initiated subsequent to a Nectar wholesaler improperly transporting a U-Haul truck containing multiple totes of untagged marijuana to one of the producer premises. Violations were found while investigating all five producer licenses, including a violation for entering marijuana items transferred from a Nectar wholesaler as inventory in Licensee’s CTS when it was actually delivered to Licensee’s unlicensed administrative offices. Other violations include camera violations, and/or failing to maintain on premises documentation for pesticides, fertilizers and agricultural chemical used in the production of marijuana. | Category III (3) | Licensee will either pay a $4,950.00 civil penalty OR serve a 30-day suspension |
| This was a minor decoy operation resulting in a sale to a minor of a $3.00 half-gram pre-rolled marijuana joint. The selling budtender relied on a door-checker who checked ID. The selling budtender claimed that she usually re-checks ID but believed the minor was of age. The door-checker made a mistake; there was no evidence of intentional sale. | Category II(b) | Licensee will pay a $3,795.00 civil penalty OR serve a 23- day suspension beginning |
| This worker permittee failed to give the Commission written notice of a felony conviction within 10 days, as required by law. Permittee is in school and relies on this employment, and was given a strong recommendation by his employer. | Category I | Permittee will pay a $750.00 civil penalty OR serve a 30-day suspension |
| This retailer allowed itself to be used by another licensee as part of a scheme to get marijuana out of the METRC Cannabis Tracking System (CTS) so the other licensee could take it to an unapproved trade show. In doing so, this retailer transferred marijuana contrary to its license privileges, and intentionally entered misleading data into CTS. Licensee admits that he erred in relying on the other licensee’s assertion that this conduct was allowable, and now reaches out to OLCC staff when he has questions about licensee requirements. | Category I (2) | Licensee will pay a $10,230.00 civil penalty OR serve a 62-day suspension |
| The Commission issued an immediate suspension based on a Category I charge for operating other than its license permits for conducting open blasting to process marijuana into butane honey oil. In addition to the charge for operating outside license privileges, the subsequent charge letter alleged Category I violations for having hemp on the premises, intentional false statement, and producing marijuana in a location that’s also a residence, as well as Category III violations for failure to tag plants and products with UID tags, and allowing consumption of alcohol and/or marijuana on the premises. | Category I (5)
Category III (3) |
License cancellation, though the licensee was permitted to try and sell its business. |
These settlements reflect that a Category I violation does not necessarily result in license cancellation. They also indicate that settlement prospects are poor when the OLCC believes a licensee is diverting marijuana into the illegal market, has defrauded its investigators, or that a licensee’s conduct is a threat to the welfare and safety of the public. Although the OLCC is willing to exercise its discretion in the appropriate case, this is determined on a case by case basis and in my experience requires the licensee to martial facts and law in support of a lesser sanction.
Where the OLCC insists on license cancellation, marijuana businesses may sometimes sell their license and/or business to a third party. But the OLCC does not give licensees an open-ended period in which to do so. Licensees who seek to sell their license in the face of a proposed cancellation must act diligently and quickly because the OLCC will set a time limit for the sale and if not complete by certain date, the license simply will be cancelled. In most circumstances, licensees may continue to operate their business during the administrative proceedings. Depending on the caseload at the OLCC, this may mean several months of continued business operations.
If find yourself on the receiving end of charging document you should:
- retain experienced counsel
- obtain the investigative report and other documents
- conduct an internal investigation
- prepare a response within the permitted time frame and
- commence settlement negotiations.
We are here to help.
The post Oregon Cannabis: Recent OLCC Settlement Trends appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/3hK69fR
via IFTTT
Sunday, July 26, 2020
Richard DeLisi, 71, suffers in a Florida prison while others make millions on marijuana
While DeLisi pushes a walker around his cell, Florida medical marijuana companies will bring in $800 million this year.
The post Richard DeLisi, 71, suffers in a Florida prison while others make millions on marijuana appeared first on Leafly.
from Leafly https://ift.tt/2WWYkvs
via IFTTT
The Roll-Up #153: All about cannabis concentrates
Oil, wax, shatter, budder, keif, hash, rosin: All this and an interview with the Dank Duchess.
The post The Roll-Up #153: All about cannabis concentrates appeared first on Leafly.
from Leafly https://ift.tt/3hD1BrH
via IFTTT
The EU May Designate CBD Foods as “Narcotics” (Yes You Read that Right.)

The craze for hemp-derived cannabidiol (CBD) extends beyond the United States and into Europe. We’ve written a good amount on the difference between how the European Union (EU) regulates CBD in contrast to the United States, and link to a number of those posts at the bottom of this page. All of that may be put on hold soon as the EU weighs new laws for CBD products.
For some background, the European Foods Safety Authority (“EFSA”) previously classified CBD as a “novel food” ingredient. A “novel food” is “food that was not used for human consumption to a significant degree within the Union before 15 May 1997, irrespective of the dates of accession of the Member States to the Union.” Pursuant to EU regulations, anyone who wishes to sell food containing a “novel food” ingredient must first secure a license from the EFSA.
Guidance issued by the EU on a plethora of various cannabinoids suggested that foods containing hemp-derived cannabinoids (and not just CBD) were considered novel foods because there has been no demonstration that they were consumed prior to the 1997 date. If something is a novel food, then certain regulatory approvals are needed to advance it in the market. Needless to say, it’s a long process, but if you want to read more about it, see the first linked post at the bottom of this page.
In mid-July 2020, it was reported (see here and here) that the EU was halting novel food applications for foods containing CBD and is apparently considering designating such CBD-bearing foods as narcotics. And here we were thinking that the United States’ policy on CBD in foods was bizarre….
According to reports, the EU notified all of the approximately 50 CBD novel foods applicants and directed them to provide feedback as to whether the substances should be designated as narcotics. Notably, this only applies to naturally occurring CBD products and an EU spokesperson reportedly noted that applications for products bearing synthetically derived CBD may still proceed. Additionally, since this only covers novel foods, this may not have an effect on CBD in other kinds of products, such as cosmetics.
We imagine the EU’s most recent change of position will be met with intense pushback from applicants, and it’s been reported that the European Industrial Hemp Association has been lobbying hard against it. Only time will tell whether the EU tries to label or regulate CBD gummies as narcotics, but in the meantime, let’s just hope the FDA’s highly anticipated regulations don’t go down the same path.
For a list of some of our earlier EU CBD posts, see:
- What To Consider Before Entering the European CBD Market
- Exporting CBD Food Just Got Harder: The European Union Makes a Move
- The Sale of CBD Foods Is Legal in the UK (For Now)
- Hemp CBD Across Europe: Germany
- Hemp CBD Across Europe: Spain
- European CBD Sales: Securing a Novel Food Authorization
The post The EU May Designate CBD Foods as “Narcotics” (Yes You Read that Right.) appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/3hEk4UY
via IFTTT
Saturday, July 25, 2020
The Philippines: Cannabis in Duterte’s Backyard?

For those of you familiar with the Philippines, you know that “President Rodrigo Duterte” and “war on drugs” are two parts of the same phrase. In the first two years of his presidency, Duterte sanctioned the killing of over 10,000 Filipinos who were connected in any way to the drug trade. But you might be surprised at the trending undercurrents within the Philippines regarding cannabis, even in light of Duterte’s strong stance against drugs.
By way of background, the Philippines is a country with a population of approximately 107 million, roughly 1/3 that of the United States population and only 20 million fewer people than Mexico’s 126 million. It is a highly religious country, with approximately 92% adhering to some form of Christianity and 6% adhering to Islam. Approximately 64% of the country speaks English, and approximately 10 million Filipinos live and work overseas, making it one of the largest diaspora populations in the world. Due to its high English speaking population, its significant diaspora in the United States, and its strong pharmaceutical industry that is already tied to the United States, the Philippines is an attractive cannabis participant, both as part of the supply chain and as a potential sales market.
Cannabis has been in serious discussion in the Philippines congress since 2014. Duterte became president in 2016, but that did not stop Filipino legislators from proposing several versions of the Philippines Compassionate Medical Cannabis Act since 2016, which would legalize a medical-only cannabis marketplace.
Earlier this year, the Philippines’ Dangerous Drugs Board (“DDB”) indicated its willingness to approve the use of medicines with CBD like Epidiolex for “medical purposes” to treat certain illnesses, as long as they contain no more than 0.1% THC. The DDB clarified that its approval removed the need for any type of medical cannabis legislation, which is not a surprise considering Duterte’s stance. In that same notice, the DDB reiterated that both recreational and medical marijuana remain illegal in the Philippines. It is interesting to note that the DDB pointed to the U.S. FDA and DEA in describing its reasoning for this position.
Philippines legislators who are cannabis advocates include Luis Ray Villafuerte from Camarines Sur, the most vocal advocate currently, who proposed HB 3961, said the legislature should act in, “creating a state agency [proposed as the Philippine Cannabis Development Authority] to oversee the development of what could be a legitimate multi-billion-dollar export industry.” Other legislators such as Panfilo Lacson and Aquilino Pimentel III have also weighed into the discussion regarding whether a medical cannabis act is necessary or the most beneficial way for the Filipino people to access cannabis and cannabis-derived medical products.
In terms of takeaways, it is clear that the Philippines is paying close attention to the U.S. FDA and DEA. Filipino legislators and regulators are both aware of the nuances in cannabis as hemp, cannabis as marijuana, and cannabinoids derived from cannabis. And Filipino legislators and economists are also acutely aware of Thailand’s head start in the region and the overall economic benefits that could come to the Philippines as a result of establishing a medical marijuana market at home and participating in the global cannabis supply chain.
Even though Duterte currently ineligible for reelection in 2022 because the Philippines no longer permits reelection for presidents, some have indicated that he will remain very politically active in the coming years, meaning an uphill battle for any type of legal cannabis market in the Philippines. But do not discount economic expedience as a driving factor for future legalization. And do not think that the Philippines is the only southeast Asian country beyond Thailand that is taking a serious look at cannabis. India is well underway, and Bangladesh will not be far behind.
For more international cannabis, check out:
- Israel: Cannabis Powerhouse
- International Cannabis: Guidance for Companies Entering the U.S. Market, Part 1
- International Cannabis: Guidance for Companies Entering the U.S. Market, Part 2 – Taxation
- International Cannabis: Guidance for Companies Entering the U.S. Market, Part 3 – State Governance
- International Cannabis: Guidance for Companies Entering the U.S. Market, Part 4 – Geography Matters
- The Rise of Cannabis Litigation Against Foreign Entities – Where Will You Litigate?
- Global Law and Business Podcast: The Uruguay Cannabis Industry
- The World Trade Organization and Cannabis
- The International Cannabis Trade: The Webinar Video Replay
- International Cannabis Continues to Look to the U.S. Market
- As China’s Hemp Industry Suffers, U.S. Hemp Growers Prepare to Pounce
- Hemp CBD Across Europe: Spain
- Hemp CBD Across Europe: Germany
- European CBD Sales: Securing a Novel Food Authorization
The post The Philippines: Cannabis in Duterte’s Backyard? appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/32ZTMIN
via IFTTT
Friday, July 24, 2020
Michigan Native American Tribe Announces Retail Partnership With Cannabis Company
from News – High Times https://ift.tt/3fY5pDr
via IFTTT
How medical marijuana and air guitar sent this ‘Hurt Locker’ hero to prison
After serving on a bomb squad in Iraq, Sean Worsley managed his PTSD with medical marijuana—until Alabama sent him to prison for it.
The post How medical marijuana and air guitar sent this ‘Hurt Locker’ hero to prison appeared first on Leafly.
from Leafly https://ift.tt/2OW6Wye
via IFTTT
Legal cannabis delivery services: Here’s what you need to know
Here’s what to expect the first time you use a legal marijuana delivery service—from prices to delivery times to tipping.
The post Legal cannabis delivery services: Here’s what you need to know appeared first on Leafly.
from Leafly https://ift.tt/33UxYfR
via IFTTT
FDA Guidance on Cannabis Research: A Glimpse of What’s To Come for CBD Products?

On Tuesday, July 21, the Food and Drug Administration (“FDA”) released draft guidance for clinical research related to the development and manufacturing of cannabis-based drugs, which gained particular interest following the legalization of hemp in December 2018.
Although the guidance does not cover other FDA-regulated products, such as hemp-derived CBD (“Hemp CBD”)-infused foods and dietary supplements, the last section of the document – Section III C. – addresses delta-9 THC and dosage calculations that may be indicative of the manner in which the FDA may propose to regulate hemp-derived finished products.
The guidance provides that those using hemp raw material in their drug development activities should follow the U.S. Department of Agriculture interim rule, or any superseding rule, for sampling and testing methods in evaluating the level of delta-9 THC. Accordingly, the agency recommends that drug approval applicants submit information, such as a certificate of analysis, indicating the percent delta-9 THC by dry weight, along with detailed descriptions of testing methods used to evaluate the level of delta-9 THC to help ensure the THC concentration doesn’t exceed 0.3 percent.
While this recommendation isn’t earth-shattering, the FDA guidance goes on to addresses an unexpected and highly debated issue: the legality of intermediate, unfinished hemp-derived drug products whose THC levels may rise above the 0.3 percent limit.
Specifically, the agency warns of the eventuality that starting materials that meet the definition of hemp may be considered Schedule I controlled substances if their THC levels were to rise above 0.3. The FDA recommends that those who handle hemp material consult with the Drug and Enforcement Agency (DEA) regarding the control status of such products that are under development.
Some manufacturing processes may generate materials, such as intermediates or accumulated by-products, that exceed the 0.3 percent delta-9 THC by dry weight threshold even if the source material or finished product does not exceed the threshold. Sponsors, investigators, and applicants who anticipate generating such intermediates or by-products that may be shipped between manufacturing sites should contact DEA for recommendations.
This suggests that the FDA may only allow hemp-derived intermediate or unfinished drug product that never exceed 0.3 percent THC on a dry weight basis to be studied and evaluated. The statement further implies that the FDA may adopt a similar position with other categories of hemp-derived products. Such an approach would be detrimental to both the hemp and Hemp CBD industry given the processing methods used to convert raw hemp into extracts and other finished products inevitably increase the THC concentration, even if fleetingly.
The FDA guidance also goes on to address methodologies that should be used to calculate delta-9 THC concentrations based on the form of the drug.
Although the composition of a cannabis-derived drugs, which the FDA plans to treat as botanical raw material, will be calculated as the amount of the compound(s) naturally present on a dry weight basis prior to extraction or other manufacturing steps, this type of dry weight calculation has limited utility for intermediates such as solutions, extracts in solution (whether aqueous or nonaqueous), and for finished products.
Consequently, the FDA recommends to calculate the delta-9 THC concentration for intermediates or finished products that contain cannabis or cannabis-derived compounds based on the composition of the formulation with the amount of water removed, including any water that may be contained in excipients (“excipients” are inactive substances that serve as vehicles or mediums for drugs or other active substances).
The guidance sets forth specific calculation methodologies for a solution-based material, including intermediate, in-process material, or final drug product, and solid oral dosage form (e.g., tablet or capsule).
It remains to be seen whether these standards would prove burdensome for hemp drug developers, but these calculation methods are particularly interesting in that they are the first practical cannabis-related guidance published by the FDA. Though drugs are regulated differently from other categories of products that fall under the authority of the FDA, these methods of calculation provide Hemp CBD stakeholders with a potential framework for calculating the delta-9 THC and CBD dosage of finished products.
All that said, this guidance is not binding, it is merely a reflection of the FDA’s current thinking on the manufacturing and testing of hemp-derived drugs. As such, it remains to be seen if the FDA’s current recommendations will become legal requirements following the 60-day public comment period.
The post FDA Guidance on Cannabis Research: A Glimpse of What’s To Come for CBD Products? appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/3fXLOmN
via IFTTT
Thursday, July 23, 2020
New York Senate Passes Bill Protecting Medical Marijuana Patients From Eviction
from News – High Times https://ift.tt/2ZSz63a
via IFTTT
Clint Eastwood Sues CBD Companies For Allegedly Stealing His Name to Sell Products
from News – High Times https://ift.tt/30FAV2T
via IFTTT
How the war on drugs killed Breonna Taylor
Breonna Taylor's death is a direct result of the war on drugs. Examine how the police harmed yet another innocent and learn how you can get justice for Breonna.
The post How the war on drugs killed Breonna Taylor appeared first on Leafly.
from Leafly https://ift.tt/2CKzzf0
via IFTTT
El Congreso de EEUU le Permite a sus Tropas Usar CBD
from News – High Times https://ift.tt/2WPJ8QI
via IFTTT
House Approves Legislation Allowing CBD Use By Military
from News – High Times https://ift.tt/3jxp13y
via IFTTT
Cannabis Cup: People’s Choice Edition To Make A Stop In Oregon
from News – High Times https://ift.tt/2CGfXc5
via IFTTT
The Netherlands and Cannabis: Legal Supply Chain Experiment Continues

Given that cannabis remains federally illegal in the United States (despite the fact that more than a majority of states have some form of cannabis legal reform), we get pretty excited when we see other countries undertake legalization experiments at the federal level. Canada was the first country to legalize commercial licensing for the cultivation, production, distribution, and sale of adult use cannabis products. Certain other countries have followed suit since Canada, and now the Netherlands is maybe throwing its hat into the ring.
If you’ve ever been to Amsterdam, you’re familiar with the extremely popular and well known coffee shops that sell cannabis (up to 5 grams) to patrons (over 18) for personal consumption on site. Like any good tourist, when I visited Amsterdam for the first time back in 2013 (after Washington and Colorado legalized cannabis for adults 21 and up), I headed straight for the coffee shops to see for myself as a U.S.-based cannabis industry attorney just how they operated under Dutch and local laws.
The Netherlands and cannabis have a pretty interesting legal relationship. Mexico gets a bad wrap because of its notorious drug cartels, but the truth is that the Netherlands isn’t that far off either as a “narco state” where the sources of cannabis cultivation and production are usually from underground, very serious criminal rings in the south. In the 1970s, the country decriminalized the use and possession of cannabis to a certain extent and since then the government has made it clear that criminal prosecution for cannabis possession, in particular, is the lowest enforcement priority and “sales” of cannabis at the coffee shops is openly tolerated by law enforcement.
As a result of the governmental view, coffee shops like those in Amsterdam proliferated across the country, selling cannabis to patrons for personal, recreational use (albeit technically illegally). The cannabis that goes to the coffee shops though comes from the illegal market and it’s not regulated, taxed, or overseen by any government body (we’ve had similar markets on a state-by-state basis in the U.S. where small amounts of cannabis for medical use, for example, are decriminalized but there’s no lawful way to actually acquire any). The kicker is that the Dutch government is well aware of the foregoing issues.
Nonetheless, things seem to be changing for the better in the Netherlands over finally (potentially) establishing a lawful cultivation and distribution network in order to supply coffee shops.
In case you missed it, late in 2019, the Dutch government decided to create and implement a four-year pilot program (via the Controlled Cannabis Supply Chain Experiment Act) in 10 cities where 79 coffee shops would be supplied exclusively by government-selected and monitored cultivators/producers of cannabis flower and hash to see how a lawful chain of cultivation through sale could be worked accordingly. The program is far from perfect–Amsterdam, Rotterdam, The Hague and Utrecht (four of the biggest coffee shop markets)–are not a part of this historic experiment, cannabis selection is going to be pretty limited where no imports are allowed, and the smaller number of coffee shop participants may not yield useful data that can be broadly applied for legislation/regulation across the country. But, it’s a start.
The most recent development in the pilot program is the country’s launching of the competitive and fairly lengthy phased application process (from July 1-28, 2020) wherein the Minister for Healthcare and the Minister for Justice and Security will select five to ten growers to supply the subject coffee shops. Similar to what we see here in the states, not just anyone is going to be allowed to participate in this experiment: would-be growers will have to meet certain criteria in order to get the gig including, but not limited to, either being a resident of the Netherlands with a company in the Netherlands or using a legal entity with a Dutch address to secure permitting, passing background checks, and submitting a comprehensive business plan (which must include a financial plan) that details the following practices, among others: record keeping, testing, meeting coffee shop demand, and compliance around quality assurance. For more details and clarification on the application process, see here (and click on the English translation if you need to).
Seems like the Netherlands is taking a page from the U.S.’s book on competitive criteria to participate in the legal cannabis industry. Barriers to entry unique to the U.S. cannabis industry typically hinge on the availability of a limited number of licenses, residency, certain levels of capitalization, experience, minimal to no criminal background, and the list goes on and on.
While the Dutch pilot program is limited in time and scope for the level of grower character and compliance it seeks to achieve, it’s a legal experiment that’s needed to happen for some time to promote public and health and safety in Holland and to see, hopefully in the long run, the minimizing effects on the illegal cannabis market. We’ll certainly be keeping our eye on how the Controlled Cannabis Supply Chain Experiment Act plays out, so definitely stay tuned.
The post The Netherlands and Cannabis: Legal Supply Chain Experiment Continues appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/3huuVki
via IFTTT
Wednesday, July 22, 2020
5 purple cannabis strains that won’t make you sleepy
Looks can be deceiving—the color of cannabis has nothing to do with its effects. Check out these beautiful purple strains that will give you a boost.
The post 5 purple cannabis strains that won’t make you sleepy appeared first on Leafly.
from Leafly https://ift.tt/30Aimgk
via IFTTT
How does cannabis get its color? Here’s why some strains turn purple
What gives cannabis its purple, blue, and red hues? The answer is anthocyanin, a family of flavonoids that gives cannabis its colorful pigments.
The post How does cannabis get its color? Here’s why some strains turn purple appeared first on Leafly.
from Leafly https://ift.tt/30A7n6M
via IFTTT
Lawsuit Filed To Block Arizona Cannabis Legalization Initiative
from News – High Times https://ift.tt/3ePiJbR
via IFTTT
Canadian Legal Cannabis Sales Show Significant Industry Spike In May
from News – High Times https://ift.tt/30ACuil
via IFTTT
FDA Releases Guidelines For Cannabis-Derived Drugs
from News – High Times https://ift.tt/3jsxa9n
via IFTTT
Colorado’s Veritas Fine Cannabis Sues Nevada Cannabis Company for Trademark Infringement

Over the last couple of years, our firm has seen a massive uptick in cannabis-related trademark litigation, and have handled many of these disputes on behalf of our clients, both as stand-alone matters and in conjunction with partnership disputes. Here are a few of the trademark disputes we’ve covered recently (our blog archives are full of these posts, if you’re interested):
- The Harvest Trademark is an Ongoing Point of Contention in the U.S. Cannabis Market
- Toys “R” Us Succeeds in Trademark Lawsuit for “Depreciation of Goodwill” Against Canadian Dispensary Herbs “R” Us
- Hemp Trademark Litigation Update: National Grange Sues Oregon-Based Hemp Grange
- Another Cannabis Trademark Dispute, Another Settlement
The latest lawsuit was filed on July 10, 2020 by Colorado-based Carrick-Harvest, LLC d/b/a Veritas Fine Cannabis (“Veritas Fine Cannabis”) against defendants Veritas Farms, Inc. and 271 Lake Davis Holdings, LLC d/b/a Veritas Farms (“Veritas Farms”), which is based in Nevada. The lawsuit alleges trademark infringement, false designation of origin, unfair competition, cybersquatting, and declaratory relieve stemming from Veritas Farms’ use of the VERITAS mark in conjunction with its cannabis products.
What makes this lawsuit interesting, and also tricky, is that it involves a licensed cannabis business in one state (Colorado) claiming trademark infringement by another licensed cannabis business in a different state (Nevada). For those who have been following our blog, you likely know that obtaining federal trademark protection is a difficult proposition for cannabis companies, due to “lawful use in commerce” being a requirement for registration with the USPTO.
In light of this difficulty, cannabis businesses have employed a two-part strategy to protect their brand assets that includes obtaining federal trademark protection for ancillary goods and services that do not run afoul of the federal Controlled Substances Act (“CSA”), as well as state trademark protection covering cannabis, cannabis goods, and any other items that are lawful under state law, but federally illegal. So, best case scenario for a state-legal cannabis business is the following:
- It possesses state trademark registrations in each state in which it legally operates, which provide protection for cannabis goods only within the borders of that state; and
- It possesses federal trademark registrations for ancillary goods and services which provide protection nationwide, but do not cover cannabis goods.
So, when a cannabis operator in one state wants to sue a cannabis operator in another state for using the same or a confusingly similar trademark, as in this case, the plaintiff must rely on federal trademark registrations that don’t cover cannabis, and state trademark registrations that don’t apply in the state in which defendant operates. It’s a difficult situation.
In this case, plaintiff has a number of active federal trademark applications (not registrations), that cover things like “providing a website containing current events news and information about cannabis, cannabis infused products and smoker’s articles,” “providing agricultural information about cannabis and cannabis strains; providing a website featuring information relating to the therapeutic benefits of cannabis; providing a website containing agricultural news and information about cannabis and cannabis,” “lighters for smokers, ashtrays,” and “providing a website containing consumer product news and information about cannabis, cannabis infused products, and smoker’s articles,” all of which are federally legal, but none of which are cannabis. (As a side note, one thing that is unclear is why these applications were filed on an intent-to-use basis when the plaintiff claims to have been using its mark in commerce since 2016.)
The plaintiff here is alleging trademark infringement by defendant based on defendant’s provision of the goods/services listed above, and also based on the contention that defendant’s cannabis goods fall within the plaintiff’s “zone of natural expansion.” This means that based on the goods and services for which plaintiff currently has federal trademark protection, it would be natural to assume that plaintiff would expand its product offerings, and therefore its trademark protection, into the same (cannabis) goods that defendant is offering.
The concept of the “zone of natural expansion” is one that virtually every cannabis company with federal trademark protection hopes to be able to rely on, but the argument is not a clear winner. Cases like this illustrate how critical it is for the industry to have access to a functioning means to protect intellectual property. Lack of access to federal trademark protection is a huge liability, and we’ll be watching to see if a decision on these issues is ultimately rendered.
The post Colorado’s Veritas Fine Cannabis Sues Nevada Cannabis Company for Trademark Infringement appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/3juZE2o
via IFTTT
Tuesday, July 21, 2020
Vending Machines Now Selling Cannabis Products In Czech Republic
from News – High Times https://ift.tt/2CqUd40
via IFTTT
Wheat Field Serves As Blank Canvas For Artist Shepard Fairey’s Hemp-Inspired Latest Work
from News – High Times https://ift.tt/2WL1GSl
via IFTTT
Shepard Fairey’s 76-acre cannabis crop art will blow your mind
The artist worked with Charlotte's Web to create the world's largest crop art installation in a Kansas hemp field.
The post Shepard Fairey’s 76-acre cannabis crop art will blow your mind appeared first on Leafly.
from Leafly https://ift.tt/30A2zyc
via IFTTT
Marihuana de ‘Tiger King’: Lo Que Necesitas Saber
from News – High Times https://ift.tt/3juLBtx
via IFTTT
Border Patrol keeps seizing legal cannabis far from border in California
And it's costing fledgling businesses—licensed by the state, and following the rules—millions of dollars.
The post Border Patrol keeps seizing legal cannabis far from border in California appeared first on Leafly.
from Leafly https://ift.tt/39iwFtZ
via IFTTT
Indiana Set the Standard for Hemp CBD Labeling, But Misses the Mark on Smokable Hemp

Indiana has long been at the forefront of hemp product regulations. Two years ago in 2018, Indiana passed a comprehensive law that regulated “low-THC hemp extracts.” At the time, state-level regulation of CBD was mostly limited to medical marijuana programs in conservative states that only allowed CBD extracts for use by medical patients. Very few states were making the distinction for CBD derived from hemp (Hemp CBD) and fewer still were imposing manufacturing, testing, and labeling requirements.
I was fascinated by Indiana’s law because it was so rare at the time. In June of 2018, I wrote about Indiana’s hemp framework for this blog, noting that the state’s robust labeling requirements were “roughly equivalent to what we see as far as packaging and labeling requirements for cannabis products in Washington, Oregon and California, the states in which our cannabis business attorneys are located.” For instance, one of Indiana’s labeling requirements is “[a] scannable bar code or QR code linked to a document that contains information with respect to the manufacture of the low THC hemp extract, including the:
(A) batch identification number;
(B) product name;
(C) batch date;
(D) expiration date, which must be not more than two years from the date of manufacture;
(E) batch size;
(F) total quantity produced;
(G) ingredients used, including the:
(i) ingredient name;
(ii) name of the company that manufactured the ingredient;
(iii) company or product identification number or code, if applicable; and
(iv) ingredient lot number; and
(H) download link for a certificate of analysis for the low THC hemp extract.”
IC-24-3-21-4(1)
As it would turn out, Indiana was setting an industry standard for Hemp CBD products. In February 2019, Kristen Nichols, editor of Hemp Industry Daily, wrote in Marijuana Business Magazine about how Utah had recently “told CBD producers they need to put a code on labels to prove the products are legal.” She went on to write:
The Beehive State was following a path similar to one blazed by Indiana, another state not exactly known for embracing progressive ideas.
When it comes to CBD, though, Utah and Indiana might be on to something.
Utah regulators were obviously paying attention to the Hoosier state. In Utah, one of the labeling requirements for “an industrial hemp product containing a cannabinoid” is a “scannable bar code, QR code, or web address” linked to a document containing a list of items identical to the list required by Indiana, except for the fact that in Utah the list of ingredients is not required in the QR-linked document. UAC R68-26-5 (5). But, Utah does require that labels comply with federal law standards, so a list of ingredients, if applicable, would have to appear on the product itself. Id. at (11).
Soon after, Louisiana and Texas would also require or permit QR codes on Hemp CBD products sold in their state. Other states continue to adopt the “Indiana-style” labeling requirements. Eventually, I believe that the FDA will impose requirements for Hemp CBD that are similar to those established in Indiana and further developed in states like Utah. Utah’s regulations are more nuanced in the sense that they impose different requirements for different products. For example, Hemp CBD vapor products must be labeled differently than products intended for ingestion.
In light of the above, the the recent 7th Circuit decision on smokable hemp is all the more meaningful. If you missed our post last week on that, the 7th Circuit lifted an injunction that prevented Indiana from enforcing Act 516 to prohibit the delivery, possession, manufacture, and sale of smokable hemp. As this litigation indicates, for better and worse, Indiana is a leader when it comes to hemp products. It’s not uncommon for a state or city to become a leader in a particular area of law. Delaware has become the jurisdiction of choice for LLCs and corporations because its laws and judges are more sophisticated and business-friendly. The DC Circuit Court is similarly advanced when it comes to questions of administrative law.
Indiana has doubled down on the ban on its smoakable hemp ban, enacting a new law, Act 335, that mirrors Act 516’s prohibition on smokable hemp, without making it unlawful for individuals to ship smokable hemp through the state. That is key, because the interstate transport of hemp is protected by the 2018 Farm Bill and lead to the now-lifted injunction. Indiana could have also prohibited Hemp CBD back in 2018, but instead, it took a nuanced and thoughtful approach to regulate the product. I have no doubt that the state could do the same with smokable hemp by requiring that sellers register in the state to obtain a license and by restricting the use of smokable hemp to those over the age of 21.
It is disappointing to see a state-leader in progressive hemp regulations take a regressive approach to smokable hemp. In my opinion, unlike low-THC extracts, smokable hemp is just a little too close to marijuana for Indiana’s liking. I have a solution though: just legalize and regulate marijuana so you do not have to worry about people smoking hemp. Unfortunately, I do not see that happening in the near term in Indiana.
The post Indiana Set the Standard for Hemp CBD Labeling, But Misses the Mark on Smokable Hemp appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/2CByVAD
via IFTTT
Monday, July 20, 2020
California Records Fewest Felony Pot Arrests Since 1954
from News – High Times https://ift.tt/3jnGs6D
via IFTTT
Study Shows Cannabis May Relieve Pain Caused By Sickle Cell Disease
from News – High Times https://ift.tt/3jlDyiF
via IFTTT
Innovative, Psychedelic Treatments In Development for Veterans
from News – High Times https://ift.tt/2OJIIHj
via IFTTT
Canada’s cannabis pardon program is failing. Here’s why
More than a year after Canada went legal, only 436 people have applied. Is the process too burdensome?
The post Canada’s cannabis pardon program is failing. Here’s why appeared first on Leafly.
from Leafly https://ift.tt/2Cngb81
via IFTTT
Is Kratom Legal?

Recently, our attorneys have seen has been a large uptick in interest, both from consumers and businesses, in ketamine and psychedelics. There has been a proliferation of ketamine clinics in the United States (see here here, and here), and dozens of jurisdictions (such as Denver and Oregon) passed or are considering passing psychedelics laws that would do everything from decriminalizing psychedelics to regulating them. But interest has not stopped there and we’ve also been fielding questions about newer or lesser known substances as well.
Today, we’re going to write about a substance that’s gotten a lot less attention: kratom. Kratom is a species of plant (Mitragyna speciosa) that is native to certain regions in Southeast Asia. Kratom derivatives have been used in a number of different formulations for human consumption, including in powder form, edible form, and for smoking. We have worked with kratom industry businesses sporadically, going back to 2015.
Advocates for kratom say that it is a natural, plant-based substance that has properties which, among other things, can assist with opioid withdrawal and addiction or pain management. Critics of kratom believe that the plant has not been sufficiently studied to be deemed safe, is in fact not safe, can cause dependence, and can have serious and potentially fatal side effects. As I’ll explain below, the federal Food and Drug Administration (FDA) and a half dozen states agree with the critics.
What makes kratom interesting is that, like hemp-derived CBD (today at least), it is not as heavily regulated as other substances (though that may of course change). Kratom is not a scheduled narcotic on the federal Controlled Substances Act (CSA). In 2016, the Drug Enforcement Agency (DEA) announced its intent to make kratom a Schedule I narcotic (alongside heroin, LSD, and cannabis), but it subsequently withdrew that position.
Today, the DEA considers kratom to be a “Drug of Concern”, along with other substances like salvia divornum. This means, according to the DEA, kratom may “pose risks to individuals who abuse [it]”. Because it’s not on any CSA schedule, kratom is not outright prohibited at the federal level (more on that below), and it’s at least theoretically possible that research can be done on it.
Notwithstanding that kratom is not a federally controlled substance, a number of states currently prohibit the substance and the list of such states could change at any time. The states that broadly prohibit kratom include Alabama, Arkansas, Indiana, Rhode Island, Vermont, and Wisconsin. Additionally, counties or municipalities within states may place additional restrictions or prohibitions on the possession, use, distribution, or manufacture of kratom.
Enter the FDA. Even in those U.S. jurisdictions where kratom isn’t expressly prohibited, sales of kratom can lead to serious penalties. The FDA may prohibit the sale of kratom products given the agency’s belief that kratom has the potential to be harmful. The FDA seized apparent kratom products on multiple occasions (for example, see here) and initiated enforcement actions while claiming that the substances were being marketed as unapproved drugs. The FDA also issued an import alert in 2019 allowing for the detention without physical examination of a host of apparent kratom products on the grounds that they were adulterated.
The FDA’s view on kratom is very different from its views on cannabidiol (CBD). The FDA prohibits many CBD products under the so-called Drug Exclusion Rule, which essentially holds that an FDA-approved substance cannot be marketed in certain products. According to the FDA, CBD cannot be added to many products because the FDA earlier approved a drug (Epidiolex) containing CBD.
The FDA’s objection to kratom isn’t based on the Drug Exclusion Rule, but rather on the substance’s potential for harm and abuse. The federal government’s enforcement position over the past few years also signals that the agency takes kratom much more seriously than it does CBD.
All this is to say that there can be significant risks for companies selling or marketing kratom products, despite the fact that it’s not outright illegal at the federal level. The regulatory pathway for kratom is by no means well-settled and there is ongoing research (for example, see here) into kratom that could lead to changes in law or enforcement priorities. We intend to keep writing on this substance, so stay tuned to the Canna Law Blog.
The post Is Kratom Legal? appeared first on Harris Bricken.
from Canna Law Blog – Harris Bricken https://ift.tt/3eLD8i3
via IFTTT